Baking Soda
Background
Baking soda is a white crystalline powder (NaHCO 3 ) better known to chemists as sodium bicarbonate, bicarbonate of soda, sodium hydrogen carbonate, or sodium acid carbonate. It is classified as an acid salt, formed by combining an acid (carbonic) and a base (sodium hydroxide), and it reacts with other chemicals as a mild alkali. At temperatures above 300 degrees Fahrenheit (149 degrees Celsius), baking soda decomposes into sodium carbonate (a more stable substance), water, and carbon dioxide.
The native chemical and physical properties of baking soda account for its wide range of applications, including cleaning, deodorizing, buffering, and fire extinguishing. Baking soda neutralizes odors chemically, rather than masking or absorbing them. Consequently, it is used in bath salts and deodorant body powders. Baking soda tends to maintain a pH of 8.1 (7 is neutral) even when acids, which lower pH, or bases, which raise pH, are added to the solution. Its ability to tabletize makes it a good effervescent ingredient in antacids and denture cleaning products. Sodium bicarbonate is also found in some anti-plaque mouth-wash products and toothpaste. When baking soda is used as a cleaner in paste form or dry on a damp sponge, its crystalline structure provides a gentle abrasion that helps to remove dirt without scratching sensitive surfaces. Its mild alkalinity works to turn up fatty acids contained in dirt and grease into a form of soap that can be dissolved in water and rinsed easily. Baking soda is also used as a leavening agent in making baked goods such as bread or pancakes. When combined with an acidic agent (such as lemon juice), carbon dioxide gas is released and is absorbed by the product's cells. As the gas expands during baking, the cell walls expand as well, creating a leavened product.
In addition to its many home uses, baking soda also has many industrial applications. For instance, baking soda releases carbon dioxide when heated. Since carbon dioxide is heavier than air, it can smother flames by keeping oxygen out, making sodium bicarbonate a useful agent in fire extinguishers. Other applications include air pollution control (because it absorbs sulfur dioxide and other acid gas emissions), abrasive blastings for removal of surface coatings, chemical manufacturing, leather tanning, oil well drilling fluids (because it precipitates calcium and acts as a lubricant), rubber and plastic manufacturing, paper manufacturing, textile processing, and water treatment (because it reduces the level of lead and other heavy metals).
Imported from England, baking soda was first used in America during colonial times, but it was not produced in the United States until 1839. In 1846, Austin Church, a Connecticut physician, and John Dwight, a farmer from Massachusetts, established a factory in New York to manufacture baking soda. Dr. Church's son, John, owned a mill called the Vulcan Spice Mills. Vulcan, the Roman god of forge and fire, was represented by an arm and hammer, and the new baking soda company adopted the arm and hammer logo as its own. Today, the Arm & Hammer brand of baking soda is among the most widely recognized brand names.
Named after Nicolas Leblanc, the French chemist who invented it, the Leblanc process was the earliest means of manufacturing soda ash (Na 2 CO 3 ), from which sodium bicarbonate is made. Sodium chloride (table salt) was heated with sulfuric acid, producing sodium sulfate and hydrochloric acid. The sodium sulfate was then heated with coal and limestone to form sodium carbonate, or soda ash.
In the late 1800s, another method of producing soda ash was devised by Ernest Solvay, a Belgian chemical engineer. The Solvay method was soon adapted in the United States, where it replaced the Leblanc process. In the Solvay process, carbon dioxide andammonia are passed into a concentrated solution of sodium chloride. Crude sodium bicarbonate precipitates out and is heated to form soda ash, which is then further treated and refined to form sodium bicarbonate of United States Pharnacopoeia (U.S.P.) purity.
Although this method of producing baking soda ash is widely used, it is also problematic because the chemicals used in the process are pollutants and cause disposal problems. An alternative is to refine soda ash from trona ore, a natural deposit.
Raw Materials
Baking soda, or sodium bicarbonate, comes from soda ash obtained either through the Solvay process or from trona ore, a hard, crystalline material. Trona dates back 50 million years, to when the land surrounding Green River, Wyoming, was covered by a 600-square-mile (1,554-square-kilometer) lake. As it evaporated over time, this lake left a 200-billion-ton deposit of pure trona between layers of sandstone and shale. The deposit at the Green River Basin is large enough to meet the entire world's needs for soda ash and sodium bicarbonate for thousands of years.
Because the synthetic process used in the Solvay method presented some pollution problems, Church & Dwight Co. Inc. is basing more and more of its manufacturing on trona mining. Another large producer of soda ash, the FMC Corporation, also relies on trona to manufacture soda ash and sodium bicarbonate. Trona is mined at 1,500 feet (457.2 meters) below the surface. FMC's mine shafts contain nearly 2,500 (4,022.5 kilometers) miles of tunnels and cover 24 square miles (62 square kilometers). Fifteen feet (4.57 meters) wide and nine feet (2.74 meters) tall, these tunnels allow the necessary equipment and vehicles to travel through them.
The Manufacturing
Process
Process
Making soda ash
- 1 Soda ash can be manufactured chemically using the Solvay process, or it can be made from trona ore. If trona ore is used, it must first be mined. After it has been brought to the surface, the trona ore is transported to a variety of processing plants. There, the ore is refined into a slurry of sodium sesquicarbonate, an intermediate soda ash product that actually contains both soda ash (sodium carbonate) and baking soda (sodium bicarbonate).
Making baking soda
- 2 Next, the intermediate soda ash solution is put into a centrifuge, which separates the liquid from the crystals. The crystals are then dissolved in a bicarbonate solution (a soda ash solution made by the manufacturer) in a rotary dissolver, thereby becoming a saturated solution. This solution is filtered to remove any non-soluble materials and is then pumped through a feed tank to the top of a carbonating tower.
- 3 Purified carbon dioxide is introduced into the bottom of the tower and held under pressure. As the saturated sodium solution moves through the tower, it cools and reacts with the carbon dioxide to form sodium bicarbonate crystals. These crystals are collected at the bottom of the tower and transferred to another centrifuge, where excess solution (filtrate) is filtered out. The crystals are then washed in a bicarbonate solution, forming a cake-like substance ready for drying. The filtrate that is removed from the centrifuge is recycled to the rotary dissolver, where it is used to saturate more intermediate soda ash crystals.
- 4 The washed filter cake is then dried on either a continuous belt conveyor or in a vertical tube drier called a flash dryer. The theoretical yield from the process, according to the Church & Dwight Company, is between 90 and 95 percent, and the baking soda manufactured is more than 99 percent pure.
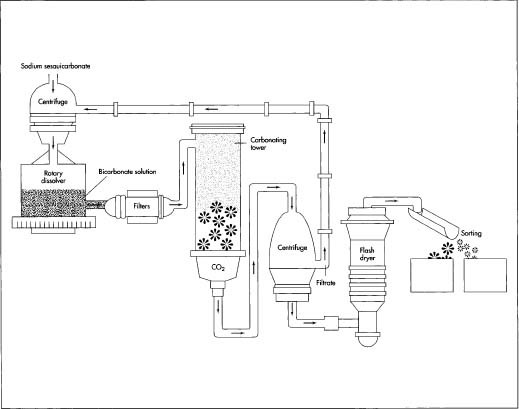
An illustration of the baking soda manufacturing process. A key step in the process occurs in the carbonating tower. Here, the saturated soda ash solution moves from the top of the tower downward. As it falls, the solution cools and reacts with carbon dioxide to form sodium bicarbonate crystals—baking soda. After filtering, washing, and drying, the crystals are sorted by particle size and packaged appropriately.
Sorting and storing the
different grades
- 5 Next, the dried crystals of sodium bicarbonate are separated into various grades by particle size. Standard grades of sodium bicarbonate and special grades are manufactured to meet customers' specific requirements, and particle size is the major determinant of grades. Powdered #1 and fine granular #2 have a wide range of uses in foods, chemicals, and pharmaceuticals. Granular grades #4 and #5 are found in foods and doughnuts, cleaning compounds, pharmaceuticals, and many other products. Industrial grade sodium bicarbonate is used in diverse applications, including oil well drilling fluids, fire extinguishing materials, and water treatment.
- 6 Each grade goes to a holding bin wherein atmosphere, carbon dioxide, and moisture content are controlled to "cure" the product. Once cured, the grades are ready to be packaged and shipped.
Quality Control
The quality of sodium bicarbonate is controlled at every stage of the manufacturing process. Materials, equipment, and the process itself are selected to yield sodium bicarbonate of the highest possible quality. According to FMC sources, when the company constructed plants, it chose materials and equipment that would be compatible with the stringent quality requirements for making pharmaceutical grade sodium bicarbonate. FMC also uses Statistical Process Control (SPC) to maintain unvarying daily quality, and key operating parameters are charted to maintain process control. Product quality parameters are recorded by lot number, and samples are kept for two to three years.
All U.S.P. grades meet the United States Pharmacopoeia and Food Chemicals Codex specifications for use in pharmaceutical and food applications. In addition, food grade sodium bicarbonate meets the requirements specified by the U.S. Food and Drug Administration as a substance that is Generally Recognized as Safe (GRAS).
The Future
At the turn of the twentieth century, 53,000 tons (48,071 metric tons) of baking soda were sold annually. While the population increased dramatically, sales by 1990 were down to about 32,000 tons (29,024 metric tons) per year. Self-rising flour and cake and biscuit mixes have decreased the demand for baking soda as an important baking ingredient. Nevertheless, demand for the product is still significant. Commercial bakers (particularly cookie manufacturers) are one of the major users of this product. One of the most important attributes of sodium bicarbonate is that, when exposed to heat, it releases carbon dioxide gas (CO 2 ) which makes the baking goods rise. Sodium bicarbonate is also used in the pharmaceutical and health industries, and it has other industrial applications as well. It therefore continues to be an important product for today and for the future.
Where To Learn More
Books
Coyle, L. Patrick, Jr. The World Encyclopedia of Food. Facts on File, 1982.
Root, Waverley and Richard de Rochemont. Eating in America: A History. William Morrow & Co., Inc., 1976.
Periodicals and Pamphlets
Grosswirth, Marvin. "The Wonders of NaHCO 3 ," Science Digest. March, 1976.
History of the Arm & Hammer Trademark. Church & Dwight Co., Inc.
Sodium Bicarbonate. FMC Corporation.
Sodium Bicarbonate — Chemical Properties, Manufacturing. Church & Dwight Co., Inc.
— Eva Sideman
Read more: http://www.madehow.com/Volume-1/Baking-Soda.html#ixzz49h8fXwdx
No comments:
Post a Comment